Description
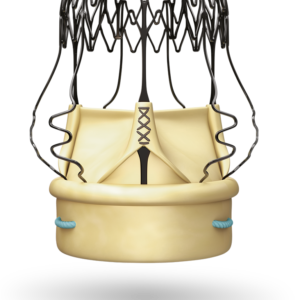
Abbott Amplatzer PFO Occluder
Key Features
- Minimally Invasive Procedure:
- Inserted via a catheter through the femoral vein, avoiding open-heart surgery.
- Short recovery time for the patient.
- Self-Expanding Nitinol Frame:
- Ensures optimal positioning and adaptation to the patient’s anatomy.
- Promotes Tissue Healing:
- The polyester fabric encourages endothelialization, sealing the PFO effectively over time.
- High Success Rate:
- Proven to significantly reduce the risk of recurrent stroke in patients with cryptogenic stroke linked to PFO.
- Long-Term Solution:
- Designed for permanent implantation, providing lifelong closure of the PFO.
Indications
- Stroke Prevention:
- For patients with a history of cryptogenic stroke where PFO is suspected as a contributing factor.
- Migraine Relief (Investigational Use):
- Emerging research suggests potential benefits in reducing migraines in certain patients with PFO.
- Divers or High-Risk Professions:
- To reduce decompression illness risks in professional divers or pilots with PFO.
Procedure Overview
- Pre-Procedure:
- Patients undergo imaging tests like echocardiography to confirm the presence and size of the PFO.
- Placement:
- A catheter delivers the occluder through the femoral vein to the heart.
- The device is positioned across the PFO and deployed, with discs anchoring securely on either side of the septum.
- Post-Procedure Care:
- Short-term anticoagulation or antiplatelet therapy is prescribed to prevent clots while the device endothelializes.
- Follow-up imaging to confirm proper placement and closure.
Clinical Benefits
- Reduces Stroke Risk:
- Effectively prevents emboli from crossing the PFO and reaching the brain.
- Minimally Invasive:
- Reduced risks and faster recovery compared to surgical options.
- Improved Quality of Life:
- Offers peace of mind for patients at risk of recurrent stroke.
- Durability:
- Designed for permanent implantation, ensuring long-term efficacy.
The Abbott Amplatzer PFO Occluder is a trusted solution for addressing PFO-related complications, offering patients a safe, minimally invasive, and effective method to reduce their stroke risk.