Description
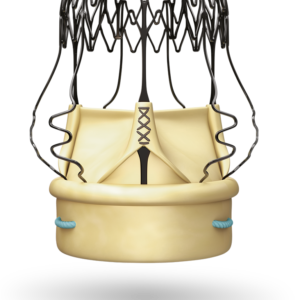
Boston Scientific Watchman FLX (Left Atrial Appendage Closure Device)
-
- Flexibility for Complex Anatomies:
- The Watchman FLX can adapt to various LAA shapes and sizes, making it suitable for a broader range of patients compared to previous models.
- Enhanced Safety:
- The fully rounded design minimizes tissue injury and complications during implantation.
- Complete LAA Sealing:
- The PET membrane ensures complete occlusion, reducing the risk of blood clots escaping the LAA.
- Improved Implantation Success Rates:
- Simplified delivery and deployment systems contribute to higher procedural success.
- Long-Term Solution:
- Offers a permanent alternative to long-term oral anticoagulants, which can pose bleeding risks.
- The Watchman FLX is an advanced Left Atrial Appendage Closure (LAAC) device designed to reduce stroke risk in patients with non-valvular atrial fibrillation (NVAF) who are unsuitable for long-term anticoagulation therapy. This innovative device permanently seals the left atrial appendage (LAA), a common site for blood clot formation in NVAF patients.
- Flexibility for Complex Anatomies: